How to Make a Solution in a Volumetric Flask
The other substance is placed in the burette burette pipette conical flask Using the pipette Method. Boil the solution you made in a stainless steel platinum or titanium evaporating dish.

Row Of Volumetric Flasks With Volume Reduction Of Color Solution Stock Illustration Download Image Now Istock
Chris the Chemist has been given a 25000 mL volumetric flask and asked to use it to make an aqueous solution of sodium chloride NaCl with a concentration of 0100 mol L-1 for a corrosion experiment.

. Prepare a 70-mgL sulfate standard solution as follows. However the standard phrase. Gently pour the solution into the dish and place it on a boiler.
The titrant reacts with a solution of analyte. Like beakers Erlenmeyer flasks are not normally suitable for accurate volumetric measurements. Transfer the sucrose into a 100 mL volumetric flask using a wash bottle to rinse any solid remaining on the weighing boat.
Use a pipet to add 70 mL of 1000-mgL sulfate standard solution into the volumetric flask. Determine the mass in grams of sodium chloride that Chris the Chemist will. One substance generally the one we dont know the concentration is put in the conical flask.
Add water to the flask until it is one-half to two-thirds full. Then re-run the. Invert flask several times to ensure uniform solution.
And glass rod to the volumetric flask. Place the NaCl in a 1-liter volumetric flask. Wash the beaker rod and funnel several times using de-ionised water from the wash bottle letting the washings go into the flask.
100-mL volumetric flask Class A 7-mL volumetric pipet Class A and pipet filler safety bulb Deionized water 1. Fill the flask to the 1 L line. If a different molarity is required then multiply that number times the molar mass of NaCl.
Transfer the solution into the volumetric flask using the funnel. Remember to fill so the bottom of the. Measure out the required amount of sucrose into a new plastic weighing boat.
Prepare this solution daily. Take 01 mL of your 1000 ppm stock and dilute it to 100 mL use a volumetric flask for accuracy and convenience. For example if you wanted a 05 M solution you would use 05 x 5844 gmol of NaCl in 1 L of solution or 2922 g of NaCl.
Remove the last drop of solution from the glass rod onto the funnel. Hot vapors from the boiling solvent keep the filter funnel warm avoiding the premature crystallization. Use the test procedure to.
Make up to the mark with distilled water using a dropping pipette for last few drops. Required to make 1000 mL of a 010 M solution. I suggest that you make a 1 ppm stock and start from there.
Make up to the mark on the volumetric flask with de-ionised water. Water boils at 212 F 100. If you dont have access to one skip this stepdo not attempt it.
Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a substance to be analyzed. Add a small volume of distilled deionized water to dissolve the salt. The hot solution is filtered through a fluted filter paper into the receiving flask.
Always conduct this step in a professional laboratory at a college university or workplace. Their stamped volumes are approximate within about 5 accuracy. Titrate solution A with solution B means that A should be in the conical flask and B should be in the burette.
Making a solution Alternatively the known mass of solid in the weighing bottle could be transferred to beaker washed and washings added to the beaker. It is measured using a volumetric pipette. To prepare roughly 1 M NaOH solution you must dissolve 20 g of NaOH in distilled water using 500 ml volumetric flask or 2 g of NaOH in distilled water using 50 ml volumetric flask.
Dilute to the mark with deionized water. Pour down the glass rod. The sodium chloride NaCl is available as an analytical reagent composed of white crystals.
A reagent termed the titrant or titrator is prepared as a standard solution of known concentration and volume.

Solved 3 Making Solutions Using Volumetric Flasks When Chegg Com

How To Use A Volumetric Flask Youtube
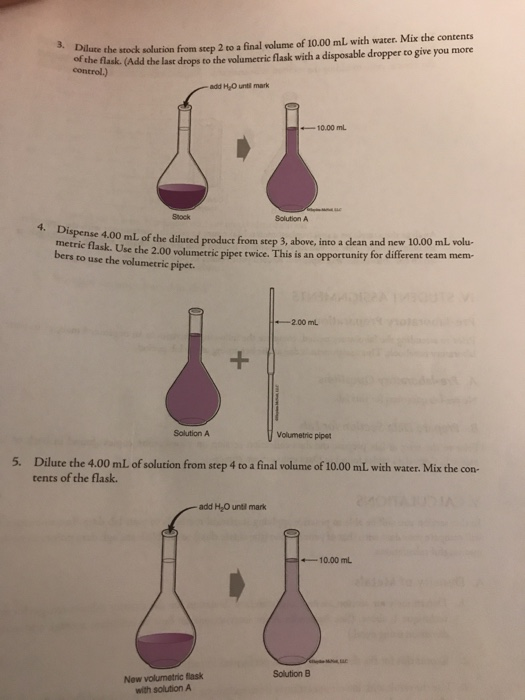
Solved Avoid Contamination Different Than Prepare The Cu 6 Chegg Com
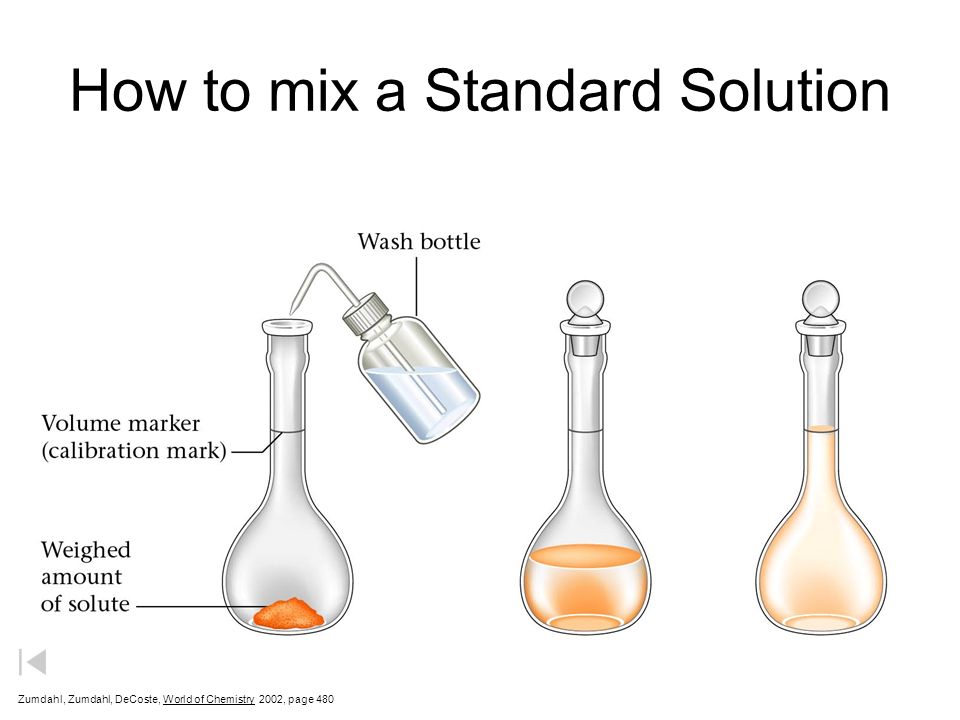
How To Mix A Standard Solution Ppt Video Online Download
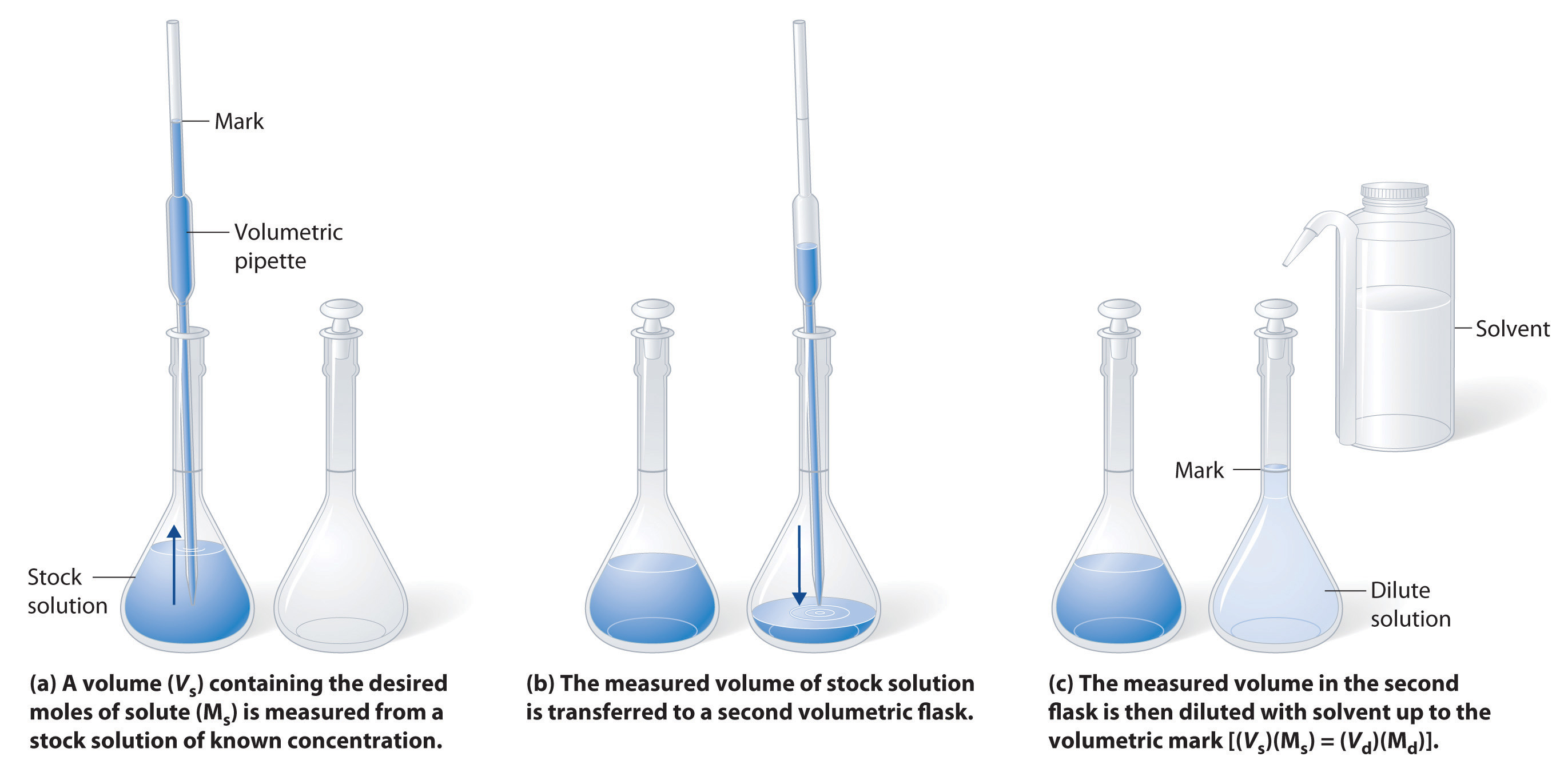
Ch104 Chapter 7 Solutions Chemistry

Volumetric Flask Borosilicate Glass 100 Ml Pack Of 2 Norchemist

Answered Draw 25 0 Ml Of Dilute With Water Until Bartleby
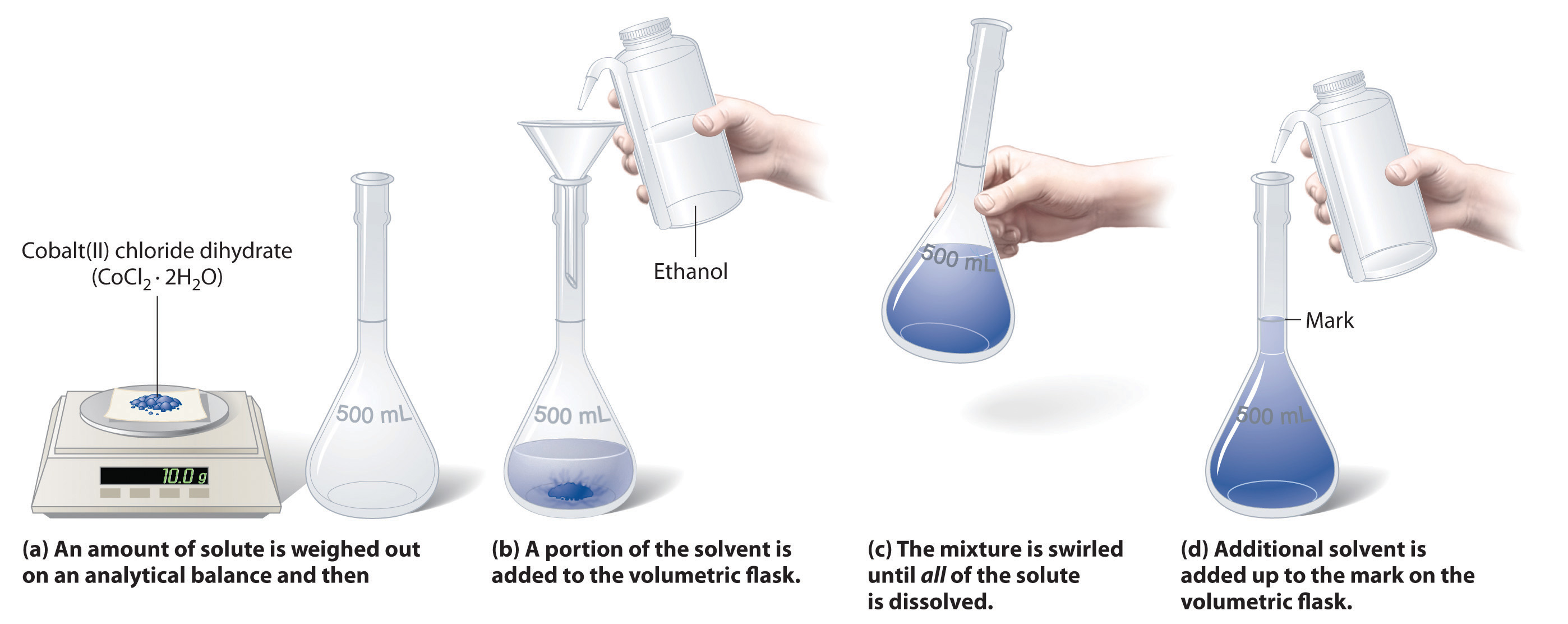
5 2 Solutions And Dilutions Chemistry Libretexts

Preparing Solutions Chemistry For Non Majors
What Are The Differences Of Using Volumetric Flask Pipette And A Measuring Cylinder Quora
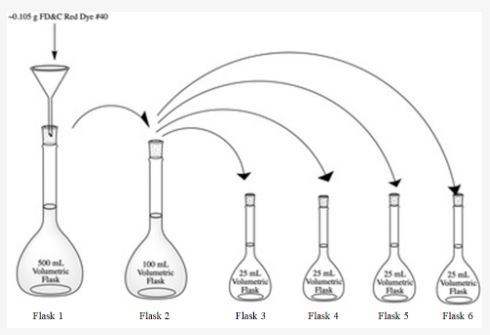
Solved 1 Following The Experimental Procedure And The Chegg Com

How To Make A Solution In A Volumetric Flask Youtube

Chapter 20 Volumetric Analysis Ppt Download

Frequently Asked Questions About Solution Preparation Carolina Com

Silver Nitrate Standard Solutions How To Make Them And Save Money Salt Lake Metals


Comments
Post a Comment